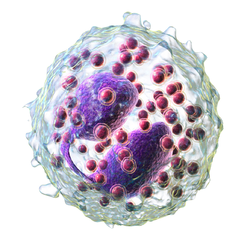
Eosinophil

Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. Along with mast cells and basophils, they also control mechanisms associated with allergy and asthma. They are granulocytes that develop during hematopoiesis in the bone marrow before migrating into blood, after which they are terminally differentiated and do not multiply. Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. Along with mast cells and basophils, they also control mechanisms associated with allergy and asthma. They are granulocytes that develop during hematopoiesis in the bone marrow before migrating into blood, after which they are terminally differentiated and do not multiply. These cells are eosinophilic or 'acid-loving' due to their large acidophilic cytoplasmic granules, which show their affinity for acids by their affinity to coal tar dyes: Normally transparent, it is this affinity that causes them to appear brick-red after staining with eosin, a red dye, using the Romanowsky method. The staining is concentrated in small granules within the cellular cytoplasm, which contain many chemical mediators, such as eosinophil peroxidase, ribonuclease (RNase), deoxyribonucleases (DNase), lipase, plasminogen, and major basic protein. These mediators are released by a process called degranulation following activation of the eosinophil, and are toxic to both parasite and host tissues. In normal individuals, eosinophils make up about 1–3% of white blood cells, and are about 12–17 micrometres in size with bilobed nuclei. While they are released into the bloodstream as neutrophils are, eosinophils reside in tissue. They are found in the medulla and the junction between the cortex and medulla of the thymus, and, in the lower gastrointestinal tract, ovaries, uterus, spleen, and lymph nodes, but not in the lungs, skin, esophagus, or some other internal organs under normal conditions. The presence of eosinophils in these latter organs is associated with disease. For instance, patients with eosinophilic asthma have high levels of eosinophils that lead to inflammation and tissue damage, making it more difficult for patients to breathe. Eosinophils persist in the circulation for 8–12 hours, and can survive in tissue for an additional 8–12 days in the absence of stimulation. Pioneering work in the 1980s elucidated that eosinophils were unique granulocytes, having the capacity to survive for extended periods of time after their maturation as demonstrated by ex-vivo culture experiments. TH2 and ILC2 cells both express the transcription factor GATA-3 which promotes the production of TH2 cytokines, including the interleukins (ILs). IL-5 controls the development of eosinophils in the bone marrow, as they differentiate from myeloid precursor cells. Their lineage fate is determined by transcription factors, including GATA and C/EBP. Eosinophils produce and store many secondary granule proteins prior to their exit from the bone marrow. After maturation, eosinophils circulate in blood and migrate to inflammatory sites in tissues, or to sites of helminth infection in response to chemokines like CCL11 (eotaxin-1), CCL24 (eotaxin-2), CCL5 (RANTES), 5-hydroxyicosatetraenoic acid and 5-oxo-eicosatetraenoic acid, and certain leukotrienes like leukotriene B4 (LTB4) and MCP1/4. Interleukin-13, another TH2 cytokine, primes eosinophilic exit from the bone marrow by lining vessel walls with adhesion molecules such as VCAM-1 and ICAM-1.When eosinophils are activated, they undergo cytolysis, where the breaking of the cell releases eosinophilic granules found in extracellular DNA traps. High concentrations of these DNA traps are known to cause cellular damage, as the granules they contain are responsible for the ligand-induced secretion of eosinophilic toxins which cause structural damage. There is evidence to suggest that eosinophil granule protein expression is regulated by the non-coding RNA EGOT.